EMPOWR™ Complex Primary Knee










EMPOWR™ Complex Primary Knee
The EMPOWR Universal Tibia and EMPOWR VVC™ Tibial Insert broaden the clinical indications of the EMPOWR Knee System™ to provide more solutions through a seamless and efficient transition from standard primary, to complex primary and revision knee arthroplasty procedures.
- Overview
- Part Numbers
- References

1. e+™ POLYETHYLENE
This knee-specific formula, blended with vitamin E and moderately cross-linked, reduces oxidation and long-term wear1
2. AUXILIARY CONSTRAINT
The Varus Valgus Constrained (VVC) insert is designed to provide the necessary support and stability in knees with supportive soft tissue deficiencies
3. WIDE RANGING GAP CORRECTION
Multiple insert thicknesses to support up to 49mm of tibiofemoral gap correction when augments are used

4. ROBUST LOCKING MECHANISMS
Dovetail and posterior captures facilitate poly insertion and securely lock insert in place. Reinforcement Pin capture, for use in 22 and 25mm inserts, provides increased static and fatigue strength in thicker inserts3
5. VERSATILE, ASYMMETRIC BASEPLATE DESIGN
Maximizes cortical coverage and prevents overhang for long-term fixation2 and can be used as a primary baseplate with the use of a stem plug, or with a stem and/or augments when indicated
6. TIERED KEEL SIZES
3 size specific lengths to closely match patient anatomy and help preserve bone
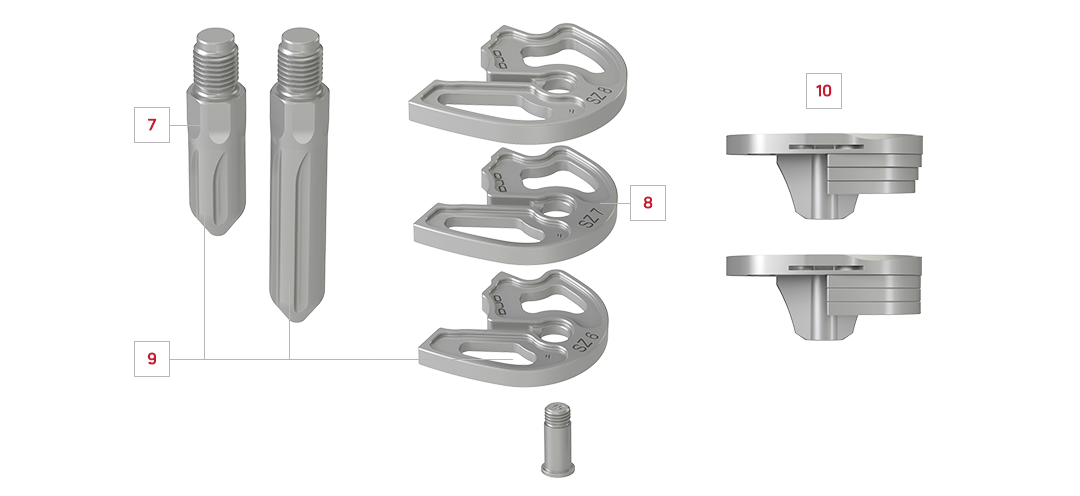
7. CEMENTED TIBIAL STEMS
Optional stems available in multiple lengths and diameters to provide additional stability
8. CEMENTED TIBIAL AUGMENTS
Optional half-block augments can be used in both medial and lateral compartments and rigidly fix to the tibial baseplate with a single, appropriate length screw
9. RIGID FIXATION
Multiple cement channels designed to allow for increased cement fixation to components
10. STACKING FLEXIBILITY
Stackable up to 15mm in blocked or tapered configurations to appropriately match tibial anatomy
| EMPOWR VVC™ TIBIAL INSERT | ||
|---|---|---|
| EMPOWR UNIVERSAL TIBIAL BASEPLATE | ||
| EMPOWR UNIVERSAL TIBIAL STEM | ||
| EMPOWR UNIVERSAL TIBIAL AUGMENT | ||
| Thicknesses | 10, 12, 14, 16, 19, 22*, 25*mm | |
| Varus/Valgus Constraint | ± 3.5° | |
| Internal/External Rotation | ± 5.5° | |
| Material | e+™ UHMWPE | |
| Sizes | 2-11 (Asymmetric) | |
| KEEL LENGTHS | 2-3 (31mm), 4-8 (35mm), 9-11 (43mm) | |
| EMPOWR Insert Compatibility | CR, 3D, PS, VVC | |
| Material | CoCr | |
| Lengths | 40, 65mm | |
| Diameters | 10, 12, 14mm | |
| Fixation | Cemented | |
| Material | CoCr | |
| Thickness | 5mm | |
| Maximum Stack Height | 15mm | |
| Fixation | Ti Screw (5, 10, 15mm) | |
| Material | Ti | |
* Reinforcement pin required
- e+™ Surgeon Testing Summary 0011110-004
- 3DKnee™ Technical Monograph 0011102-004
- EMPOWR VVC™ Locking Mechanism Testing PR17-063-01